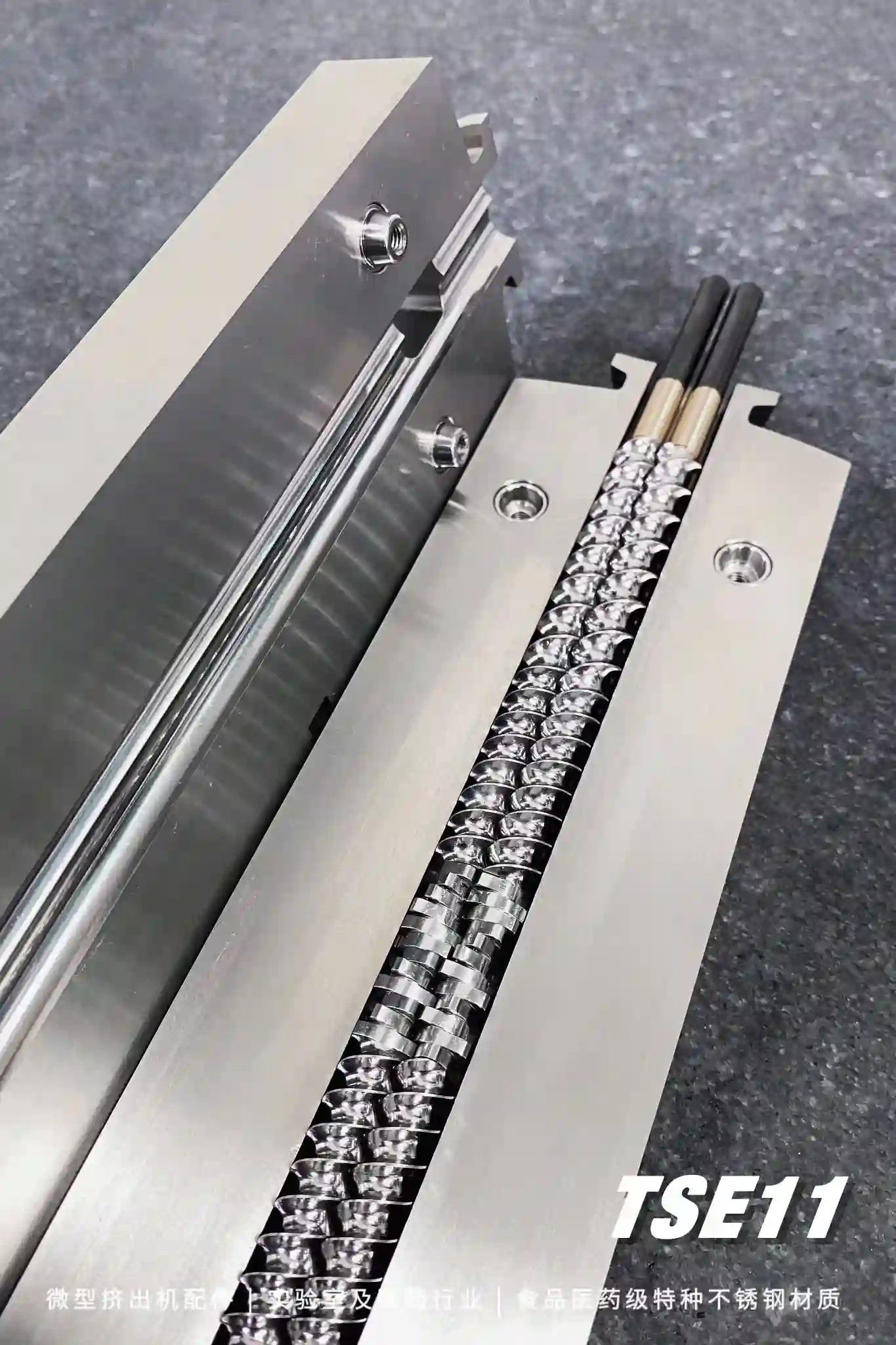
Xinda Hot Melt Extruder Machine: Clamshell Barrel
Hot-melt extrusion pharmaceutical is a proven manufacturing process that complies with FDA Process Analytical Technology (PAT) goals for designing, analyzing, and controlling manufacturing process programs.
This technology can produce granules, pellets, immediate-release tablets, controlled-release tablets, and Pellicle. At present, many drugs have been approved for marketing in foreign countries using hot melt extrusion pharmaceutical technology.
split barrel design easy to disassemble and clean
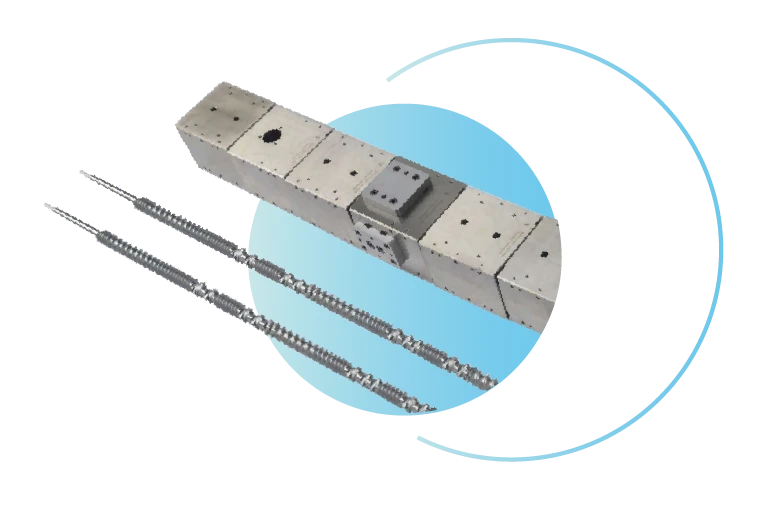
Multi-stage temperature
control system
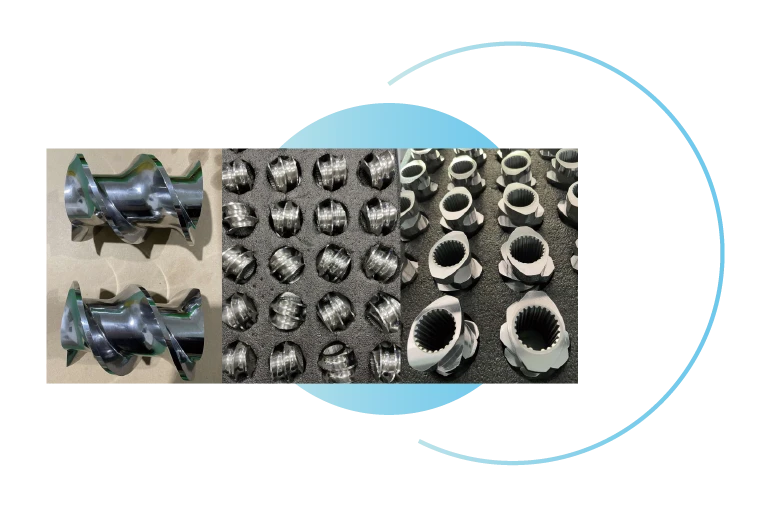
Modular screw
combination mechanism
Comply with GMP, FDA, EU and other industry regulations

Customized Software complies with 21 CFR Part 11 standards
Statistics show that 40% of the drugs sold globally are poorly soluble drugs, and the proportion of poorly soluble drugs among the drugs under research is as high as 90%. Solubility has become a key factor affecting the development and application of such drugs. With the upgrading of the industry and the advancement of production technology, the “serial production” method may be gradually replaced by “continuous production”. Have her cake, and eat it, too — a technology that can not only improve the solubility of poorly soluble drugs but also realize continuous production mode – hot melt extruder machine, has emerged.
Xinda is a manufacturer specializing in the research and manufacture of compounding extruders. For the pharmaceutical industry, we have developed 11mm and 20mm hot melt extruder machine.


| Model | PSHJ-11 | PSHJ-16 | PSHJ-20 | PSHJ-35 | PSHJ-50 |
|---|---|---|---|---|---|
| Capacity(kg/h) | 0.02~2 | 0.02-5 | 2-10 | 5-20 | 40-150 |
| Screw diameter(mm) | 11 | 16 | 21.7 | 35 | 50 |
| Length/Diameter(L/D) | 30~40 | 30~42 | 30~42 | 30~42 | 30~42 |
| Maximum Screw Speed(rpm) |
1000 | 600 | 600 | 600 | 600 |
| Single shaft Output Torque(Nm) | 7 | 16 | 32~43 | 147~294 | 438~875 |
| Total Power(kw) | 1.5 | 2 | 4~5.5 | 18.5~37 | 55~110 |
| Power Supply | 380/50HZ Three-phase five-wire |
380/50HZ Three-phase five-wire |
380/50HZ Three-phase five-wire |
380/50HZ Three-phase five-wire |
380/50HZ Three-phase five-wire |
| Total Weight(≈kg) | ≈200 | ≈300 | ≈800 | ≈900 | ≈1200 |
Various models and sizes of accessories are available
Feeder
Volumetric feeder/Gravimetric feeder/Forced volumetric feeder
Conveyor belt
water cooling/air cooling laboratory/production
Cutter
Compact size customized to meet different granulation requirements
Product Quality Index
Energy Generation

Working Principle
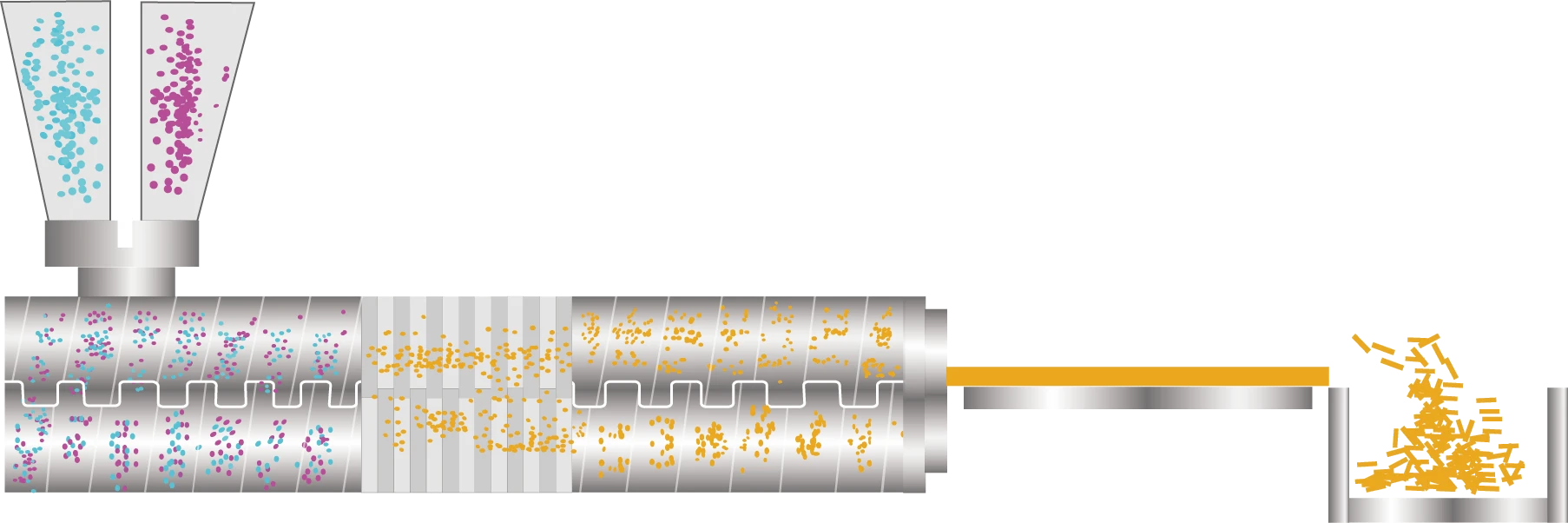
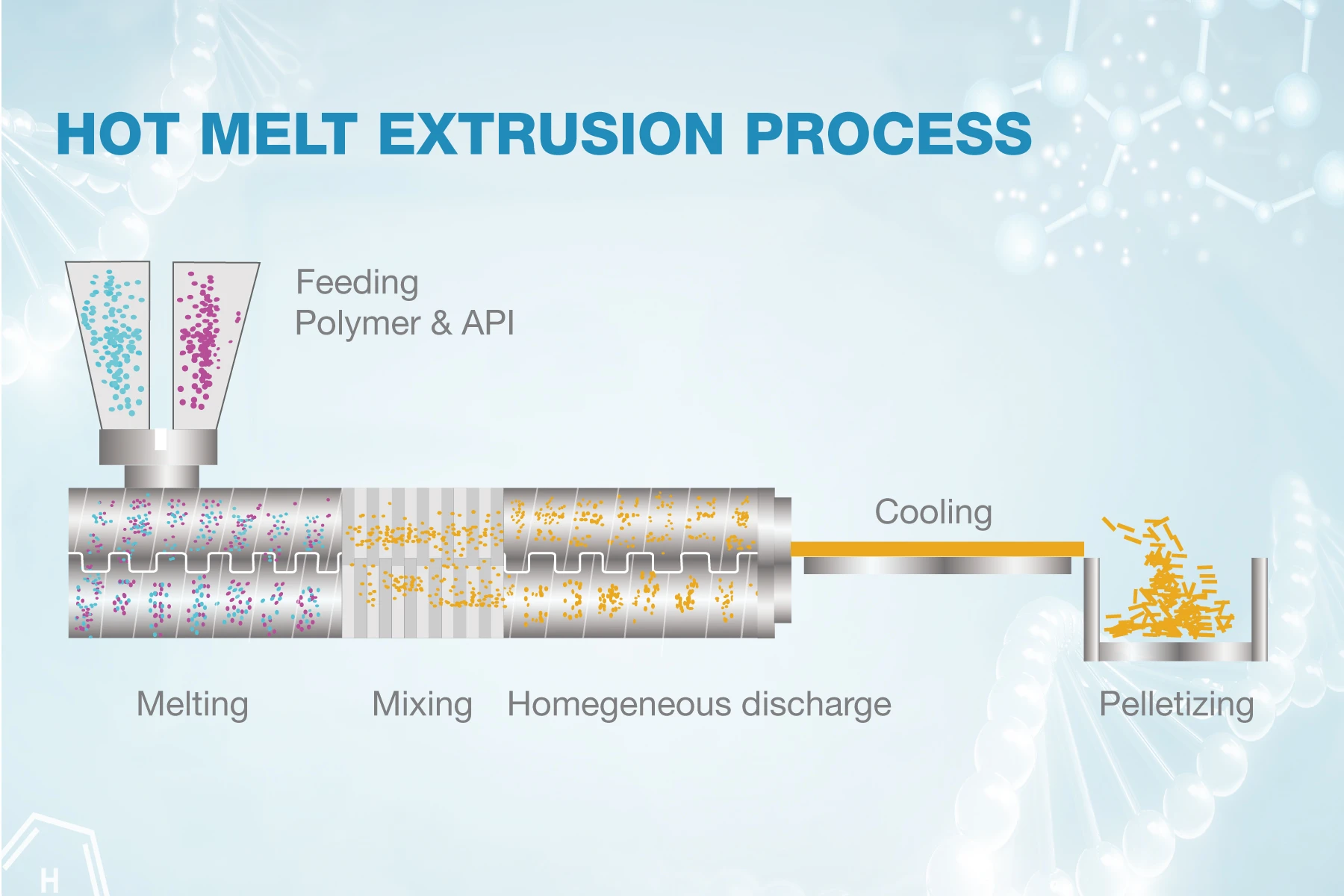
Hot melt extrusion pharmaceutical a technology that mixes drugs and polymers and other excipients in a molten state, and extrudes them at a certain pressure, speed, and shape to form products.
After the powder raw materials and auxiliary materials are fully mixed, they are evenly transported to the feed port through the feeding system, and then the API and drug carrier are evenly transported to the temperature-controlled barrel by the twin screw. The barrel is equipped with threaded elements, which perform different unit operations from the feeding position to the die head in sequence. The material moves forward under the conveying action of the threaded elements and melts or softens in the melting section. The melt is evenly mixed under the action of the mixing elements and finally extruded from the die at a certain pressure, speed and shape.
Feed the API and polymer into extruder
Under the mechanical force of the extruder and the heating of the barrel, the material melts or softens in a certain temperature range
Through the action of strong shearing and mixing by the screw, the multi-phase material undergoes symmetrical exchange and infiltration during the period, and finally it is highly uniformly dispersed in a single-phase
Remove moisture or other solvents from the material
Through screw conveying and pressurization, and further mixing at the same time, the final material is smoothly extruded through the die. According to the requirements of the dosage form, the shape of the extrudate can be changed arbitrarily through the die
After the material is extruded from the extruder, it can be cooled and shaped by air cooling, water cooling, cooling rolls, etc., and finally crushed, pelletized, or rolled according to requirements
Equipment
There are two main types of hot melt extruder machine, plunger type, and screw type. Plunger extruders are gradually eliminated due to their weak mixing ability. Screw extruders are divided into single-screw, twin-screw and multi-screw. Single-screw and twin-screw extruders are most widely used in the formulation field.
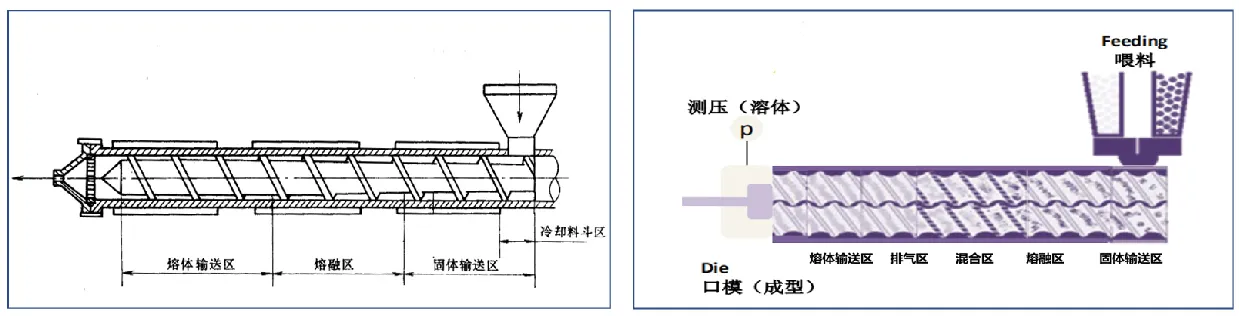
The single-screw extruder is an integral structure, which consists of cooling hopper area, solid conveying area, melting area, and melt conveying area. The twin-screw extruder is a combined structure, which consists of five parts: solid conveying zone, melting zone, mixing zone, exhaust zone and melt conveying zone.
Advantages
Hot melt extrusion pharmaceutical provides sufficient mixing and shearing. The shearing force comes from the meshing elements, and the drug can be highly mixed and uniformly dispersed in the carrier material. The dissolution rate of the drug is greatly improved, and a highly mixed and uniformly dispersed shaped product can be obtained, which can also improve the release of the drug in vivo. In order to meet the needs of different compounds and preparations, hot melt extrusion technology has many advantages:
> Use a twin-screw feeder to ensure uniform and stable feeding;
> Use segmented electric heating to ensure uniform and stable heating without temperature contamination;
> Combined twin-screw design allows you to freely select thread elements and thread lengths according to material characteristics;
> The extrusion die aperture can be selected with different apertures and different shapes of die heads according to needs;
> Cooling and crushing devices of different structures can be configured according to different material characteristics;
> Quick design, simple installation and disassembly, easy to clean;
> Customized services can be provided according to customer needs;
> Comply with industry regulations such as GMP, FDA, and EU;
> High-quality DQ, IQ, and OQ verification document system.
Application


Nirvadipine
Sporanox
Tacrolimus
Ibuprofen
Clotrimazole
Belsonara
Zoladex
Onmel
Ozurdex
Zithromax
Vlekira Pak
partners












Exhibition