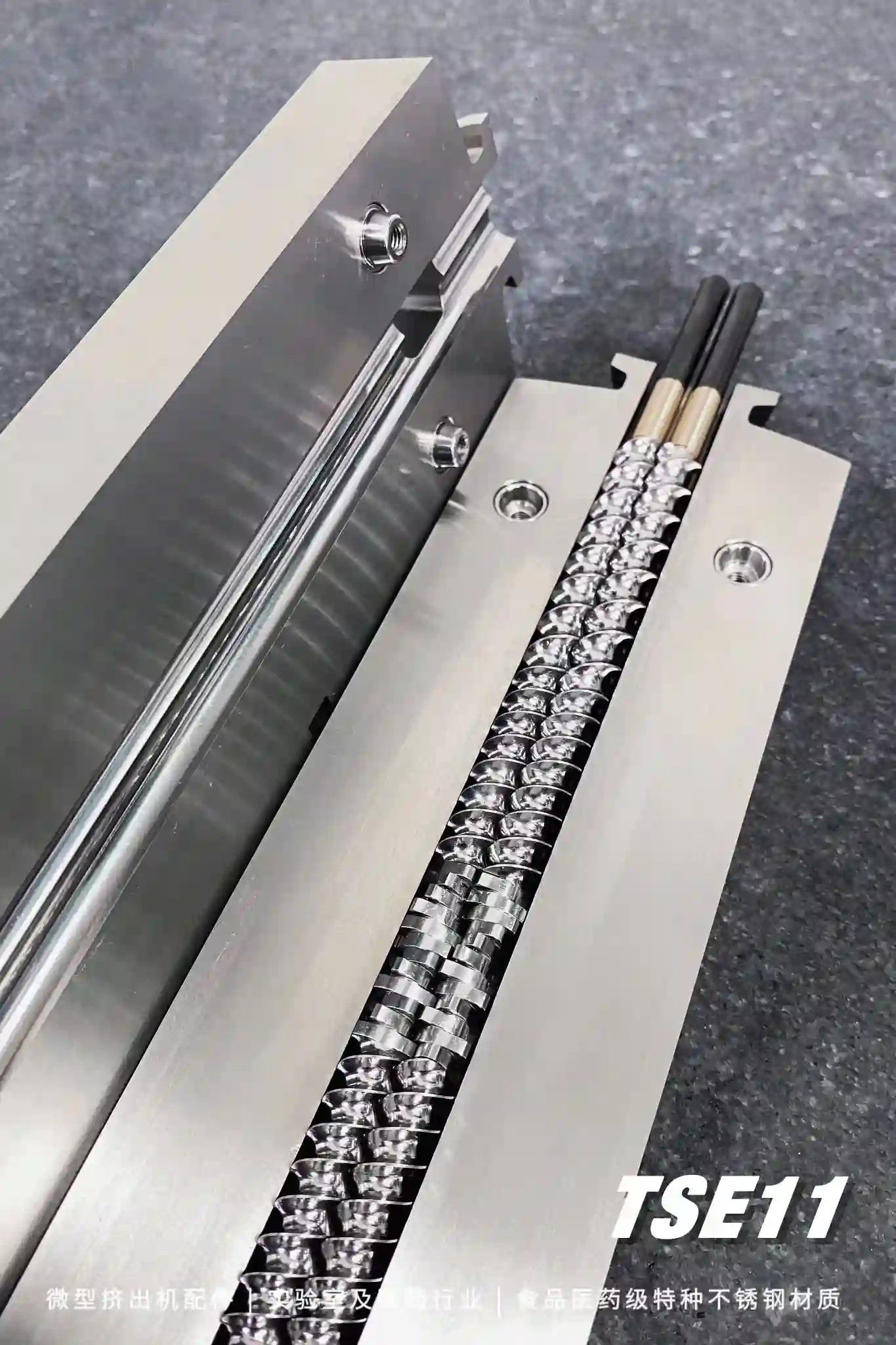
Hot-melt extrusion is a proven manufacturing process that complies with FDA Process Analytical Technology (PAT) goals for designing, analyzing and controlling manufacturing process programs.
This technology can be used to produce granules, pellets, immediate-release tablets, controlled-release tablets, and Pellicle. At present, many drugs have been approved for marketing in foreign countries using HME technology.
Statistics show that 40% of the drugs sold globally are poorly soluble drugs, and the proportion of poorly soluble drugs among the drugs under research is as high as 90%. Solubility has become a key factor affecting the development and application of such drugs. With the upgrading of the industry and the advancement of production technology, the “serial production” method may be gradually replaced by the “continuous production”. Have her cake, and eat it, too — a technology that can not only improve the solubility of poorly soluble drugs but also realize continuous production mode – hot melt extrusion technology, has emerged.

Technical Principle
Hot-melt extrusion is a technology that mixes drugs and polymers and other excipients in a molten state, and extrudes them at a certain pressure, speed, and shape to form products.
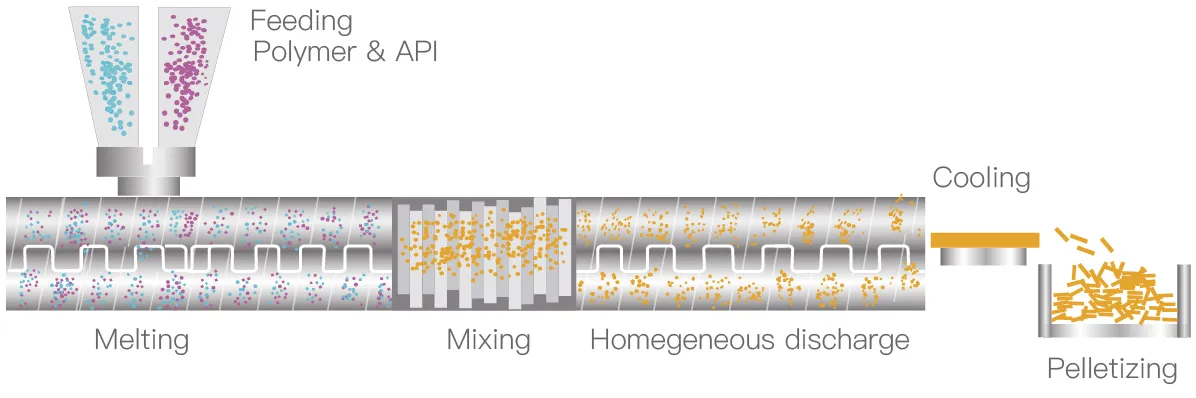
Feed the API and polymer into extruder
Under the mechanical force of the extruder and the heating of the barrel, the material melts or softens in a certain temperature range
Through the action of strong shearing and mixing by the screw, the multi-phase material undergoes symmetrical exchange and infiltration during the period, and finally it is highly uniformly dispersed in a single-phase
Remove moisture or other solvents from the material
Through screw conveying and pressurization, and further mixing at the same time, the final material is smoothly extruded through the die. According to the requirements of the dosage form, the shape of the extrudate can be changed arbitrarily through the die
After the material is extruded from the extruder, it can be cooled and shaped by air cooling, water cooling, cooling rolls, etc., and finally crushed, pelletized, or rolled according to requirements
Equipment
There are two main types of hot melt extrusion equipment, plunger type and screw type. Plunger extruders are gradually eliminated due to their weak mixing ability. Screw extruders are divided into single-screw, twin-screw and multi-screw. At present, single-screw and twin-screw extruders are most widely used in the formulation field.
The single-screw extruder is an integral structure, which consists of cooling hopper area, solid conveying area, melting area, and melt conveying area. The twin-screw extruder is a combined structure, which consists of five parts: solid conveying zone, melting zone, mixing zone, exhaust zone and melt conveying zone.
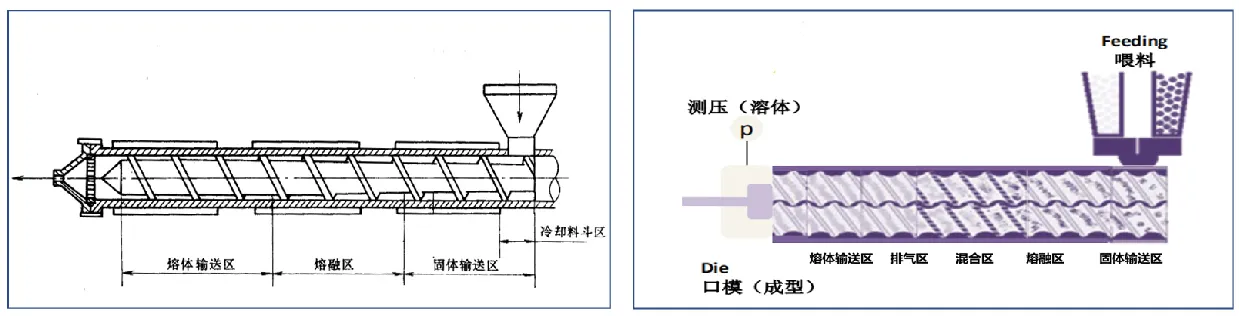
Advantages of twin-screw extruder
The residence time is short, generally between 1 and 10 minutes. The distribution range is narrow
Under the action of high shear and kneading force, the material mixing effect is better
The two screws mesh with each other and scrape each other, which has high self-cleaning ability and less material waste
Strong controllability, continuous operation for extrusion and mixing process, the screw in each section can be matched arbitrarily, and the shape of the die can also be changed according to requirements
Distributive mixing and dispersive mixing are interlaced, and the drug and poly materials are mixed more uniformly
Advantages
Hot-melt extrusion technology provides sufficient mixing and shearing. The shearing force comes from the meshing elements, and the drug can be highly mixed and uniformly dispersed in the carrier material. The dissolution rate of the drug is greatly improved, and a highly mixed and uniformly dispersed shaped product can be obtained, which can also improve the release of the drug in vivo. In order to meet the needs of different compounds and preparations, hot melt extrusion technology has many advantages:
> Poor API stability:
Polymer + API, with no water to avoid API hydrolysis
Process: The melting temperature is controllable, select the appropriate temperature to prevent API thermal degradation
> API bitter tasty:
Add taste-masking agent to change the crystal form of bitter API
> API low solubility:
Add polymer excipient carrier, reduce particle size to form microcrystals, or amorphous solid dispersions, increasing drug solubility
> Poor compressibility:
Forms a solid dispersion and improves compressibility
> Remaining solvent:
There is no need to add organic reagents during the process
> Amplify repeatability:
The same equipment can be selected for the small test and the medium test, and the repeatability is good during the enlargement process
> Batch:
Continuous production, no batch limit
> Quality uniformity:
Continuous production, uniform product quality
> Targeting:
Targeted drugs can be developed to reduce toxicity and increase efficiency
> Release rate:
Through polymer selection and process optimization, immediate release and sustained release drugs can be produced
> Dosage form:
It can meet the process requirements of multi-dosage forms, such as oral solid preparations, oral patches and implants, etc.
> Equipment requirements:
Small footprint, strong space controllability and low cost
Application
Hot-melt extrusion is a validated manufacturing process that meets the FDA’s Process Analytical Technology (PAT) goals for designing, analyzing, and controlling manufacturing process programs. This technology can be used to prepare granules, pellets, immediate-release tablets, controlled-release tablets and films, etc. At present, many drugs have been approved for marketing in foreign countries using HME technology.
Everolimus Tablets
Lynparza
Nirvadipine
Sporanox
Tacrolimus
Zithromax
Vlekira Pak
Isoptin
Nimodipine
Fenoglide
Ibuprofen
Clotrimazole
Eucreas
Belsonara
Palladone
Zoladex
Kaletra
Norvir
Cesamet
Fenoglide
Onmel
Ozurdex
NuvaRing
Opana ER
Exhibition