

Hot Melt Extrusion in Pharmaceutical Industry
Hot Melt Extrusion (HME) pharmaceutical extruder is a technology for processing polymeric materials above the glass transition temperature (Tg). Enabling thermoplastic binders and/or mixing of polymers and reactive compounds at the molecular level. In recent years, pharmaceutical extruder has been widely used for solubility-enhancing applications of polymer-based amorphous solid dispersions. If premise is that the API is thermally stable.

Unique advantages
Pharmaceutical extruder offers many advantages over other pharmaceutical processing techniques. During hot melt extrusion technique, molten polymers can function as thermal binders and act as drug depots and/or drug-release retardants upon cooling and solidification.

Principle
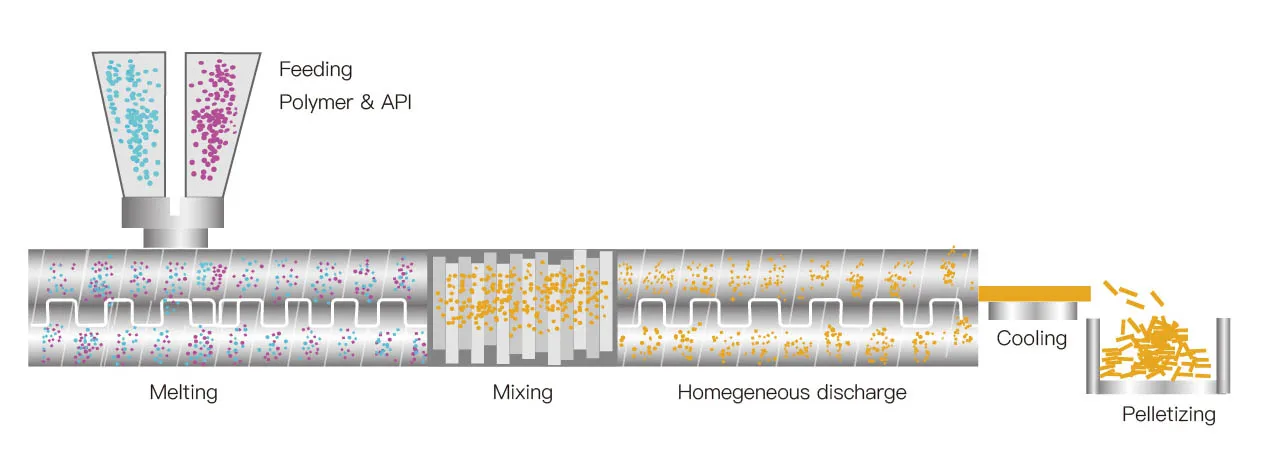
During the hot melt extrusion process, the raw and auxiliary materials are added into the feed hopper through the quantitative feeding device, and then uniformly conveyed into the barrel through the twin screws. The screw heats in stages, heats up step by step, melts the material step by step, and realizes co-melting in the middle section. At the same time, under the action of strong shear force, it disperses, distributes and mixes, and then further carries out vacuum devolatilization to remove the degraded small molecular substances, and moisture from the material, and finally extrudes the material from the die head by extrusion of the extruder screw.
Characteristic:
> Feedding System
The mixture is then subjected to heat and pressure, which melts the polymer and activates any curing agents or cross-linking agents.
> Heating method:
The upper and lower cylinders are designed to be electrically heated in sections at the same time, with uniform heating.

> Temperature Control
Multi-segment temperature control system (± 0.5 ℃ within the set value range): the temperature control feedback system is adopted to ensure the stability and reproducibility of the hot melting process.
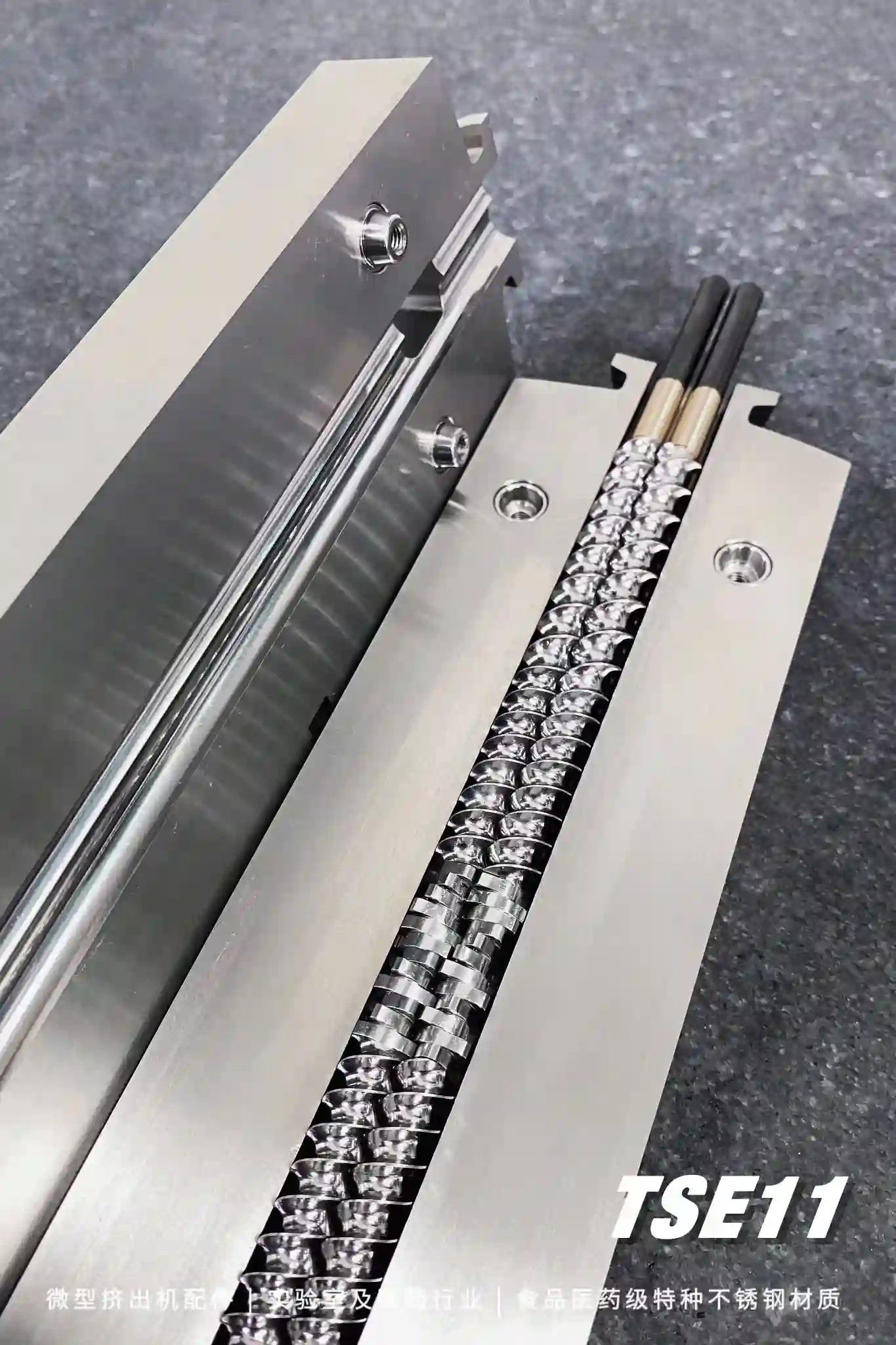
> Combined twin-screw:
The twin-screw adopts the form of multi-section splicing, which integrates mixing, pressurization, melting, extrusion and cooling to ensure the complete integration of main medicine and auxiliary materials.

> Split barrel design:
Which can be opened in a few minutes, makes product conversion, equipment maintenance and new product development very convenient.
> Multi-stage exhaust:
Multi-stage exhaust holes are designed to effectively exhaust air and water in molten materials, reduce extrusion pressure and improve product quality.
> Complies with GMP standards:
It iss easy to disassemble and clean, has three-level password authority, process parameters are automatically controlled by PLC, all data are automatically stored online, and can be exported from USB flash disk.
> Compact fuselage design:
Small footprint, and a complete production device with a total length of less than 3 meters (including upstream and downstream auxiliary machines)
Application
The hot melt extrusion technique is a validated manufacturing process that meets the FDA’s Process Analytical Technology (PAT) goals for designing, analyzing, and controlling manufacturing process programs. Extrusion in pharmaceutical industry can prepare granules, pellets, immediate-release tablets, controlled-release tablets, and films. At present, many drugs have been approved for marketing in foreign countries using HME technology.


Everolimus Tablets
Lynparza
Nirvadipine
Sporanox
Tacrolimus
Zithromax
Vlekira Pak
Isoptin
Nimodipine
Fenoglide
Ibuprofen
Clotrimazole
Eucreas
Belsonara
Palladone
Zoladex
Kaletra
Norvir
Cesamet
Fenoglide
Onmel
Ozurdex
NuvaRing
Opana ER












Exhibition
















